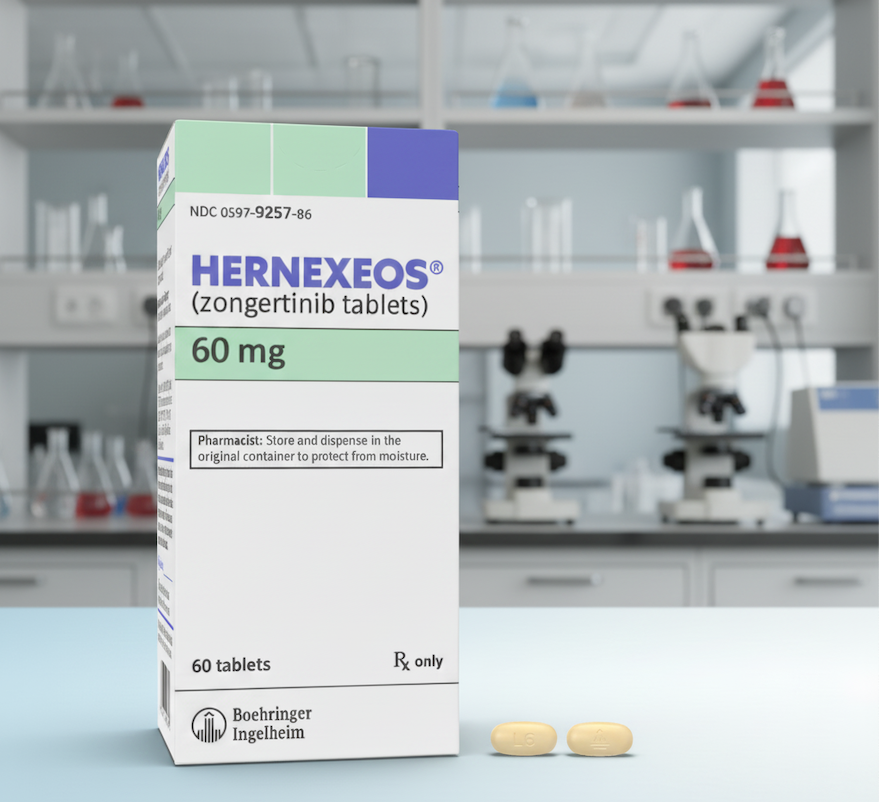
The FDA has granted accelerated approval to zongertinib (Hernexeos) for adults with advanced non-squamous non-small cell lung cancer (NSCLC) carrying HER2 tyrosine kinase domain mutations who have previously received systemic therapy. Approval was based on results from the Beamion LUNG-1 trial, which showed significant response rates and durable benefit in patients treated with the drug.
On August 8, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to zongertinib, marketed as Hernexeos by Boehringer Ingelheim, for certain patients with advanced non-small cell lung cancer (NSCLC). The approval covers adults with unresectable or metastatic non-squamous NSCLC whose tumors carry specific activating mutations in the HER2 (also known as ERBB2) tyrosine kinase domain, as confirmed by an FDA-approved test, and who have already received prior systemic therapy.
At the same time, the FDA also approved the Oncomine Dx Target Test, developed by Life Technologies Corporation, as a companion diagnostic. This test is designed to identify HER2 tyrosine kinase domain mutations in patients, making them eligible for treatment with zongertinib.
The approval was supported by results from the Beamion LUNG-1 trial (NCT04886804), an open-label, multi-center study that evaluated zongertinib in patients with HER2-mutated, unresectable or metastatic non-squamous NSCLC. The trial assessed objective response rate (ORR) and duration of response (DOR) as its primary measures, using blinded independent review based on RECIST v1.1 criteria.
Among 71 patients who had previously received platinum-based chemotherapy but had not been treated with a HER2-targeted tyrosine kinase inhibitor or an antibody-drug conjugate, the objective response rate was 75 percent, with 58 percent of patients maintaining a response lasting at least six months. In a second group of 34 patients who had already been treated with platinum-based chemotherapy and a HER2-targeted antibody-drug conjugate, the objective response rate was 44 percent, with 27 percent achieving a response lasting at least six months.
The prescribing information for Hernexeos highlights warnings and precautions for potential risks, including liver toxicity, reduced heart function, interstitial lung disease or pneumonitis, and embryo-fetal toxicity.
Dosing of zongertinib is determined by body weight. Patients weighing less than 90 kilograms are recommended a daily oral dose of 120 milligrams, while those weighing 90 kilograms or more are prescribed 180 milligrams daily. The drug can be taken with or without food and is continued until disease progression or unacceptable side effects occur.
Full prescribing information will be made available through the FDA’s Drugs@FDA resource.
What does it mean for me?
Zongertinib, which is marketed under the brand name Hernexeos®, is an innovative oral medication representing a significant advancement in the field of precision oncology. It functions as a targeted therapy, specifically a kinase inhibitor designed to block the activity of a protein known as HER2 (Human Epidermal Growth Factor Receptor 2). In certain cancers, mutations in the HER2 gene lead to an overactive form of this protein, which fuels tumor growth and proliferation. Zongertinib's mechanism is to precisely inhibit these signals, thereby helping to control the cancer.
For lung cancer patients, the approval of Zongertinib offers a crucial new option, particularly for adults with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors carry specific HER2 activating mutations and who have already received a prior systemic therapy. This provides a much-needed treatment for a patient population with limited alternatives. The development of Zongertinib underscores the importance of biomarker testing, as patients must be tested to confirm they have the specific genetic mutation that the drug targets, ensuring it is given to those most likely to benefit. The drug's approval was based on promising clinical trial data that showed a significant number of patients experienced a positive response.
As a treatment, Zongertinib is administered in tablet form and is taken orally once per day. Patients can take the medication either with or without food. The prescribed dosage is based on body weight, with a 120 mg dose recommended for individuals weighing less than 90 kg and a 180 mg dose for those weighing 90 kg or more. It is important that the tablets are swallowed whole and are not split, crushed, or chewed. Patients typically continue this treatment regimen until their disease progresses or they experience unacceptable toxicity.
This article is for informational purposes only and does not constitute medical advice. The content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read in this article.
Source: Bristol Myers Squibb. (2025). HERNEXEOS® (zongertinib) Prescribing Information.
Press Release
https://www.boehringer-ingelheim.com/us/media/press-releases/hernexeos-zongertinib-tablets-gains-accelerated-approval